Abstract
Introduction: Patients (pts) with Epstein Barr virus-positive (EBV+) diffuse large B-cell lymphoma (DLBCL), not otherwise specified (NOS), have a poorer prognosis than pts with EBV-negative DLBCL. The incidence of EBV+ DLBCL is likely underestimated, as EBER testing is not routinely performed and there is no consensus definition on a cutoff for EBER positivity. CAR T-cell therapy (CAR-T) has revolutionized the treatment landscape of relapsed/refractory large B-cell lymphoma. We sought to review the frequency of EBV + DLBCL among patients treated with CD19 CAR-T at our center and to report their outcomes.
Methods: We retrospectively reviewed charts of patients treated with CD19 CAR-T cell products (axicabtagene ciloleucel, tisagenlecleucel, lisocabtagene maraleucel) for the treatment of EBV + DLBCL pts between January 1, 2018 and May 30, 2022, followed until death or last follow-up. EBV status was determined by EBER in situ hybridization on paraffin embedded tissues. Patients were included in the analysis if EBER highlighted many lymphoma cells, referred as EBV positivity.
This study was approved by the local Institutional Review Board and conducted according to Good Clinical Practice. The main objectives of the analysis were describing pt demographics, clinical characteristics and outcomes; pathology results and estimated survival outcomes. Descriptive statistics including mean, standard deviation, median, and range for continuous variables such as age, frequency counts and percentages for categorical variables are reported. Survival outcomes were calculated from CAR-T infusion date. Kaplan-Meier method was used to estimate overall survival and progression free survival with patients followed until death and censored at last follow up. Cytokine release syndrome (CRS) and immune effector cell-associated neurologic syndrome (ICANS) were graded using ASTCT criteria (Lee et al, BBMT 2019) and CARTOX criteria before May 2019.
Results: There were 218 pts during this time frame that had CD19 CAR-T, and only 9 patients were identified to have EBER(+) lymphoma cells. In the EBV + cohort, the median age was 47 years (range, 20-81) and included 5 (55%) pts with DLBCL-NOS, 3 (30%) pts with transformed follicular lymphoma, and one (15%) pt with high-grade B-cell lymphoma. The median number of prior lines of therapy was 2 (range, 1-5). No pts in the cohort had prior autologous stem cell transplant (Auto sCT) for DLBCL due to chemorefractory disease. Two patients had a history of classic Hodgkin lymphoma (CHL) treated with ABVD. ECOG performance status at the time of CAR-T infusion was 0 in 5 pts and 1 in 4 pts. Seven pts did not require bridging therapy. Four of 6 pts assessed had CD30(+) neoplasms. Cell-of-origin assessment was available: 4 (70%) neoplasms had a germinal center B cell-like immunophenotype, 3 (30%) a non-germinal center B cell-like immunophenotype and 2 were unclassified.
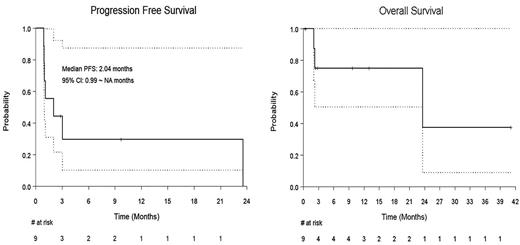
The complete response rate was 55.6% (95% CI: 21.2% ~ 86.3%) at 30 days of CAR-T infusion. The median event-free survival time was 2.04 months (95% CI: 0.99~NA months). Alive pts were censored at last follow-up date. The median OS time was 23.6 months (95% CI: 23.6~ NA months) with a median follow-up of 9.69 months (95% CI: 2.4 ~ NA months). Two pts who achieved complete remission on day 30 PET/ CT died while in remission, one patient was noted to have hemophagocytic lymphohistiocytosis and died from its complications, while another pt, who had prior Auto sCT for CHL, developed prolonged cytopenia and died from its complications. ICANS (all grades) occurred in 5 (55%) pts. Severe ICANS (grade ≥3) occurred in 3 (30%) pts. Five (55%) pts had CRS, with only 1 grade ≥3. A total of 3 pts died during the follow up period and 3 of the 6 alive pts had lymphoma progression.
Conclusion
We present a retrospective analysis of CD19 CAR-T treated pts with a diagnosis of EBV+DLBCL, NOS in this single institution series. Based on our analysis, limited by small cohort size, CD19 CAR-T appeared to be an effective therapy for EBV+ DLBCL, NOS with response rates similar to what is reported in the literature for all DLBCL patients. It is paramount to include EBER testing on all biopsy samples to understand the responses and challenges of CD19 CAR-T in this rare disease subtype. CAR T-cell therapy may be a viable treatment option for these pts, however careful pt selection will be key to mitigate adverse event risk and for eliciting durable response.
Disclosures
Nair:Incyte Corporation: Honoraria. Nastoupil:BMS, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, Genmab, Janssen, IGM Biosciences, Novartis, Takeda: Research Funding; ADC Therapeutics, BMS, Caribou Biosciences, Epizyme, Genentech/Roche, Gilead/Kite, Genmab, Janssen, MEI, Morphosys, Novartis, Takeda: Honoraria; Genentech/Roche, MEI, Takeda: Other: DSMC. Fowler:Celgene: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Roche: Consultancy, Research Funding; BostonGene Corporation: Current Employment, Other: Leadership. Lee:Takeda: Research Funding; Seagen: Research Funding; Octernal Therapeutics: Research Funding; Celgene: Research Funding; Briston-Myers Squibb: Research Funding; Janssen: Honoraria; Guidepoint Global: Honoraria; Deloitte: Honoraria; Olson Research: Honoraria; Korean Society of Cardiology: Honoraria; Curio Science: Honoraria; Cancer Experts: Honoraria; Aptitude Health: Honoraria; Pharmcyclics: Research Funding; Century Therapeutics: Membership on an entity's Board of Directors or advisory committees. Steiner:Seagen: Research Funding; BMS: Research Funding; GSK: Research Funding; Rafael Pharmaceuticals: Research Funding. Strati:Roche Genentech: Consultancy; Hutchinson MediPharma: Consultancy; ADC Therapeutics: Consultancy, Research Funding; TG Therapeutics: Consultancy; Kite Gilead: Consultancy; Astrazeneca Acerta: Research Funding; ALX Oncology: Research Funding; Sobi: Research Funding. Nieto:Astra Zeneca: Research Funding; Affimed: Other: Scientific advisory Board, Research Funding; Secura Bio: Research Funding. Chihara:AstraZeneca: Honoraria; Eisai: Honoraria. Wang:Vinverx: Research Funding; Molecular Templates: Research Funding; Meeting Minds Experts: Honoraria; Juno Therapeutics: Consultancy, Research Funding; Loxo Oncology: Research Funding; Genmab: Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; IDEOlogy Health: Honoraria; LLC TS Oncology: Honoraria; Eastern Virginia Medical School: Honoraria; Pepromene Bio: Consultancy; Dava Oncology: Honoraria; Celgene: Research Funding; Genentech: Consultancy, Research Funding; Medscape: Honoraria; VelosBio: Consultancy, Research Funding; Oncternal: Consultancy, Research Funding; BioInvent: Consultancy, Honoraria, Research Funding; Deciphera: Consultancy; InnoCare: Consultancy, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Kite Pharma: Consultancy, Honoraria, Research Funding; Leukemia & Lymphoma Society: Consultancy, Honoraria; Lilly: Consultancy, Research Funding; Merck: Honoraria; Milken Institute: Consultancy; MJH Life Sciences: Honoraria; Moffit Cancer Center: Honoraria; OncLive: Honoraria; Physicians Education Resources (PER): Honoraria; Practice Point Communications (PPC): Honoraria; Studio ER Congressi: Honoraria; Oncology Specialty Group: Honoraria; BeiGene: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Acerta Pharma: Honoraria, Research Funding; AbbVie: Consultancy. Jain:Pharmacy Times: Membership on an entity's Board of Directors or advisory committees; Aptitude Health: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Research Funding; Beigene: Research Funding; Kite Pharma: Consultancy, Research Funding; Eli Lilly and Company: Consultancy, Honoraria. Pinnix:Merck Inc: Research Funding. Green:Kite/Gilead: Research Funding; Sanofi: Research Funding; Abbvie: Research Funding; Daiichi Sankyo: Consultancy, Honoraria; Monte Rosa Therapeutics: Honoraria; Tessa Therapeutics: Honoraria; KDAc Therapeutics: Current holder of stock options in a privately-held company; Allogene: Research Funding. Flowers:Gilead: Consultancy, Research Funding; Karyopharm: Consultancy; Pharmacyclics/Janssen: Consultancy; SeaGen: Consultancy; Spectrum: Consultancy; 4D: Research Funding; NPower: Current holder of stock options in a privately-held company; Acerta: Research Funding; Adaptimmune: Research Funding; Allogene: Research Funding; Amgen: Research Funding; Cellectis: Research Funding; EMD: Research Funding; Guardant: Research Funding; Iovance: Research Funding; Janssen Pharmaceutical: Research Funding; Kite: Research Funding; Morphosys: Research Funding; Pfizer: Research Funding; Pharmacyclics: Research Funding; Sanofi: Research Funding; Takeda: Research Funding; TG Therapeutics: Research Funding; Xencor: Research Funding; Ziopharm: Research Funding; Burroughs Wellcome Fund: Research Funding; Eastern Cooperative Oncology Group: Research Funding; National Cancer Institute: Research Funding; V Foundation, Cancer Prevention and Research Institute of Texas: CPRIT Scholar in Cancer Research: Research Funding; Genmab: Consultancy; Genentech/Roche: Consultancy, Research Funding; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Denovo Biopharma: Consultancy; Celgene: Consultancy, Research Funding; BeiGene: Consultancy; Abbvie: Consultancy, Research Funding; Bayer: Consultancy, Research Funding. Vega:Crisp Therapeutics: Research Funding; Allogene: Research Funding; Geron Corporation: Research Funding. Ahmed:Chimagen: Consultancy, Research Funding; Xencor: Research Funding; Seagen: Research Funding; Merck: Research Funding; Myeloid Therapeutics: Consultancy; Servier: Membership on an entity's Board of Directors or advisory committees; Tessa Therapeutics: Consultancy, Research Funding. Westin:Kite, a Gilead Company: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; MorphoSys/Incyte Corporation: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding; MonteRosa: Consultancy; Calithera: Consultancy, Research Funding; Iksuda: Consultancy; Merck: Consultancy; Abbvie/GenMab: Consultancy; SeaGen: Consultancy. Neelapu:Bio Ascend: Consultancy, Honoraria; Medscape: Consultancy, Honoraria; Poseida: Research Funding; Aptitude Health: Consultancy, Research Funding; Bluebird Bio: Consultancy, Honoraria; Unum Therapeutics: Consultancy, Honoraria, Other: Personal fees, Research Funding; Calibr: Consultancy, Honoraria, Other: Personal fees; Adicet Bio: Consultancy, Honoraria, Other: Personal fees, Research Funding; Legend Biotech: Consultancy, Honoraria, Other: Personal fees; Precision Biosciences: Consultancy, Honoraria, Other: Personal fees, Research Funding; Incyte: Consultancy, Honoraria, Other: Personal fees; Cell Medica/Kuur: Consultancy, Honoraria, Other: Personal fees; Allogene Therapeutics: Consultancy, Honoraria, Other: Personal fees, Research Funding; Pfizer: Consultancy, Honoraria, Other: Personal fees; Celgene: Consultancy, Honoraria, Other: Personal fees, Research Funding; Novartis: Consultancy, Honoraria, Other: Personal fees; Bristol Myers Squibb: Consultancy, Honoraria, Other: Personal fees, Research Funding; Merck: Consultancy, Honoraria, Other: Personal fees, Research Funding; Kite: Consultancy, Honoraria, Other: Personal fees, Research Funding; Cellectis: Research Funding; Karus Therapeutics: Research Funding; Acerta: Research Funding; Takeda Pharmaceuticals: Patents & Royalties: related to cell therapy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal